Covid-19 Testing Program
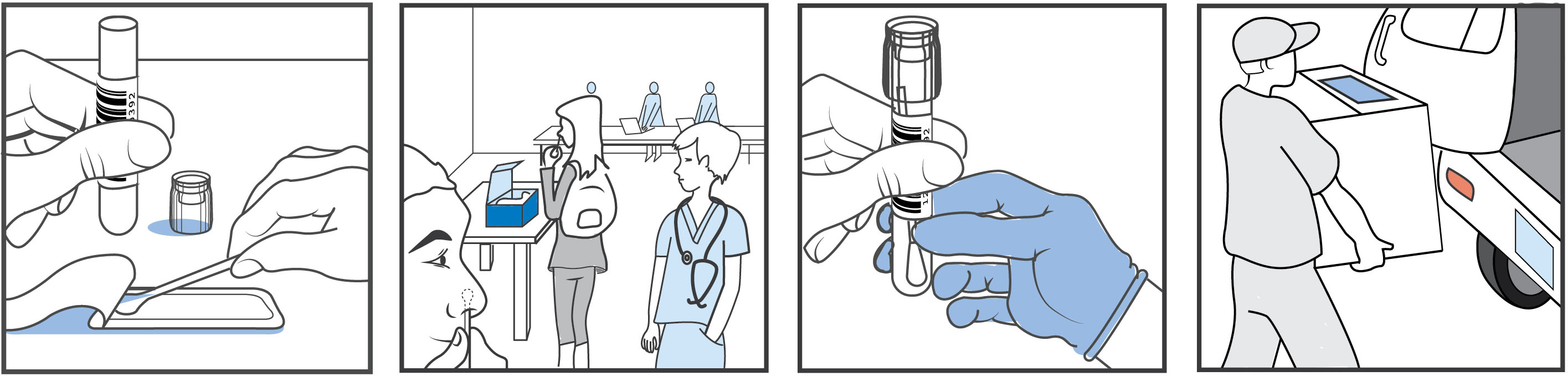
The Clinical Research Sequencing Platform (CRSP) and Broad have a strong interest in helping nursing homes, local communities and New England colleges and universities that wish to operate in a safe manner. To serve this public goal, CRSP has established a Covid-19 Testing program, providing a plan for testing at regular intervals. Because the majority of transmissions occur from people who didn't know they were infected, simply relying on symptoms is not an effective way of detecting infections. It is important to detect infections before there's been much opportunity for transmission in order to keep a community safe (in addition to decreasing spread through measures such as masks and physical distancing). Broad has received FDA Emergency Use Authorization (EUA) for Observed Self-Swab Nasal Collection , Unobserved Self-Swab Nasal Collection, and Pooled Testing.
To be part of the program, an organization is required to have a medical professional with an NPI number (physician, PA, NP) to request tests, an established regular testing cadence, and will be required to sign a Material Services Agreement (MSA) outlining terms of service, statement of work and payment details.
Costs of the program will be on a per-test basis and will include all materials for tests including tubes and swabs, and integration with Broad's systems for ordering and results reporting.
Organizations will be responsible for costs associated with testing sites logistics, staff and management.
Test turnaround time: within 24 hours of receipt of samples at the Broad CRSP lab.